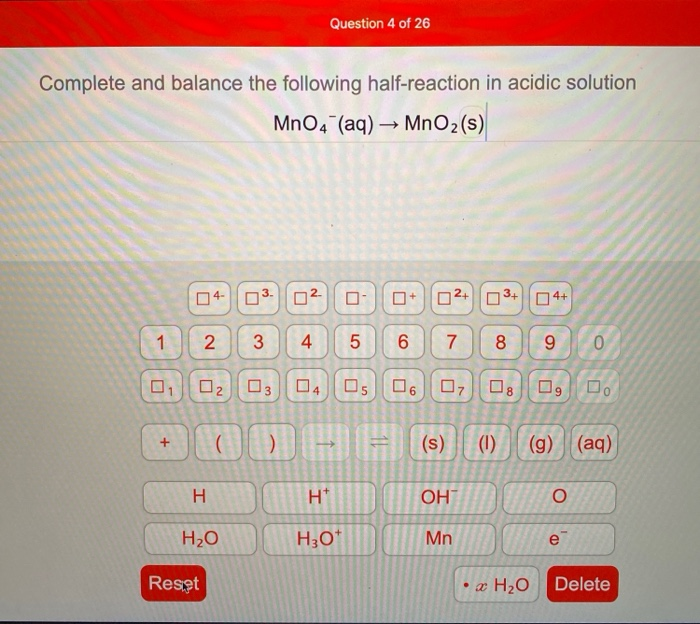
Complete and Balance the Following Half-reaction in Acidic Solution Mno4-
M nO 4 8H 5e M n2 4H 2Ol ii For each half-equation charge and mass are balanced ABSOLUTELY and thus it reflects stoichiometry. You may reference this Periodic Table and these steps to help complete the problems.
Solved Complete And Balance The Following Half Reaction In Chegg Com
First Write the Given Redox Reaction.
. Sign In to Tutor. Complete and balance the following half-reaction in acidic solution MnO4 aq MnO2 s Complete and balance the following half-reaction in basic solution 103- aq 10- aq. SO3 2- H2O _ SO4 2- _.
Sign In to Writing Essays Science. Part A NH4 MnO4- NO3- Mn2 acidic solution. Balance the following half reaction in acid solution.
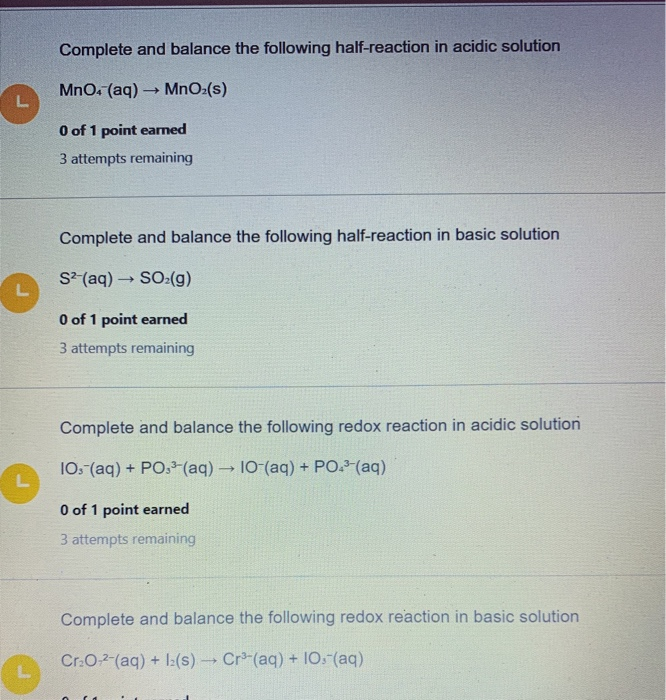
Complete and balance the following half-reaction in acidic solution MnO4 aq MnOzs 0 of 1 point earned 3 attempts remaining Complete and balance the following half-reaction in basic solution S2 aq SO2g O of 1 point earned 3 attempts remaining Complete and balance the following redox reaction in acidic solution 103-aq PO3- aq 10-aq. They undergo the above neutralization reaction. Record all of your solutions.
SO3 2- _ SO4 2- _. MnO₄ ----- MnO₂ Reduction I -----I₂ Oxidation Step3. In this activity you will practice balancing overall redox reactions in acidic and basic solutions.
Add 4 molecules of water on the left hand side to balance the oxygen. The balanced equation for the reaction in acidic solution is 14 Haq 2 Mn2aq 5 NaBiO3s 2 MnO4aq 5 Bi3aq 5 Naaq 7 H2Ol There. ChemistryQA LibraryComplete and balance the following half-reaction in acidic solution MnO4-aq.
MnO4 - 4 H _ MnO2 2 H2O _. The solution that results will appear. Separate into Half Equations.
Identify Oxidation and Reduction half Reaction. Balance the atoms undergoing change in the Oxidation number. To do this you will apply the half-reaction method for balancing redox equations.
Now balance hydrogen atoms by adding hydrogen ions on opposite to the side where water molecule are present 1. The complete and balance the equation for this reaction in acidic solution is given by. MnO4 - _ MnO2 2 H2O _.
Complete and balance each of the following half-reactions steps 25 in half-reaction methodMnO4aq Mn2aq in acidic solutionOpenStax is a registe. Find an answer to your question Complete and balance the following half-reaction in acidic solution mno4 ---. Use water to balance O.
Oxidation reaction of chromium to chromate in acidic medium. TO produce a balanced equation we adds i and ii in such a way as to remove the. Sign In to Solutions.
MnO4Mn2aq MnO4Mn23e MnO48H5eMn24H2O MnO48HMn24H2O MnO48HMn24H2O5e none of the above. Use H to balance H. MnO₄ I ----- MnO₂ I₂.
Balancing a redox reaction in basic aqueous solution in ten easy steps. MnO4 - _ MnO2 _. Mno2 babij5566 babij5566 2 days ago Chemistry High School Complete and balance the following half-reaction in acidic solution mno4 --- mno2.

Solved Question 4 Of 26 Complete And Balance The Following Chegg Com

Solved Complete And Balance The Following Redox Reaction In Chegg Com
Belum ada Komentar untuk "Complete and Balance the Following Half-reaction in Acidic Solution Mno4-"
Posting Komentar